what is the electron configuration of ag|Iba pa : Cebu Mar 23, 2023 Orphanage Directory.org is all about orphanages in Iloilo City & around the world. Basically it is online directory of orphanages worldwide, volunteer opportunities, mentorship programs and how you as an individual can help in Iloilo City. Our mission of Orphanage Directory.org portal is to make common online platform for connecting volunteers & .
PH0 · silver electron configuration
PH1 · ground state electron configuration ag
PH2 · Iba pa
Exact time in Stockholm time zone now. Official Stockholm timezone and time change dates for year 2024.
what is the electron configuration of ag*******Mar 23, 2023
We first need to find the number of electrons for the Ag atom (there are 47 electrons) using the Periodic Table. When we write the configuration, we'll put all 47 .
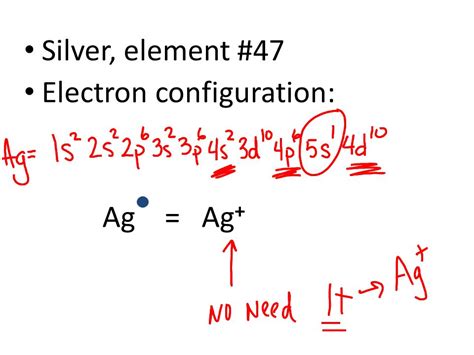
g. ? Solution. Verified by Toppr. The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of .Ag. Silver. 47. 107.868. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. .what is the electron configuration of ag Updated on February 01, 2021. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron .
what is the electron configuration of ag Iba paThe arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that .What is the electron configuration and orbital diagram of: Na + P 3– Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated .November 21, 2022 by Darshana Fendarkar. The electronic configuration of periodic elements shows the total number of electrons arranged in their atomic orbital. Let us .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 6.29) are not technically transition elements. However, the term is frequently used to refer to the entire d block .The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore the electron configuration for silver must end as 4 s 9. This notation can be written in core notation or noble gas .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the . In order to form the Ag+, an electron would be removed from the 5s sublevel, So the electron configuration of a Ag+ ion would be [Kr]4d10. "Ag": " [Kr]4d"^10"5s"^1" "Ag"^ (+)": " [Kr]4d"^10" The ground state electron configuration of "Ag" is " [Kr]4d"^10"5s"^1". In order to form the "Ag"^+, an electron would be removed from .
Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting point: 961.9 ℃. Density: 10.49 g/cm 3 . The order of filling the orbitals with electrons in the Ag atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9 But in reality, one electron .
Iba pa Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Silver (Ag) [Kr] 4d 10 5s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1: 2, 8, 18, 18, 1: 48: Electron configuration of Cadmium (Cd)The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. The equation is: 1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p. The concept of electronic configuration has replaced the older concept of valency and valence . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
The Ag( +I) oxidation state can be rationalized on this basis. Answer link. Where is your Periodic Table? For "silver", Z=47. It is thus 11 protons removed from the last Noble Gas, which is "krypton", Z=36. And thus the electronic configuration of silver metal is: [Kr]4d^ (10)5s^1. The Ag (+I) oxidation state can be rationalized on this basis.
There are several important exceptions to the general pattern for electron configurations of the elements. In this video we will look four different exceptio.

The electronic configuration of periodic elements shows the total number of electrons arranged in their atomic orbital. Let us see the electronic configuration of Ag. Electronic configuration of Ag is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. Silver is the transition metal atom its symbol is Ag. It is the 47 th periodic table element, . So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 .
Check me out: http://www.chemistnate.com
Comprehensive information for the element Silver - Ag is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 1; Electrons per Energy Level: 2,8,18,18,1 Shell Model .
Silver (Ag) has an electron configuration of [Kr] 4d 10 5s 1. The element is much more stable and has a lower energy when the 4d orbital is filled, so one electron is placed there, rather than in the 5s orbital. When it is ionized, the electron is removed from the outermost shell, which is the 5s orbital. So the electron configuration for Ag .
It determines every single chemical property of an element. - Ag is an element with the atomic number 47. It is a d-block element of group 11 and period 5. It is a transition metal. - As it is d-block element, the general configuration is given as. $ (n-1)d^ { (1-10)}ns^ { (0-2)}$. The electronic configuration of Silver would be. orbital. electron shell. octet. valence electron. subshell. electronic configuration, the arrangement of electrons in orbitals around an atomic nucleus. The electronic configuration of an atom in the quantum-mechanical model is stated by listing the occupied orbitals, in order of filling, with the number of electrons in each orbital .Depict the ground-state electron configuration for the atom by using the condensed spdf notation with a noble-gas-core abbreviation. Mo; 1. give the electron configuration of the ground state of antimony, using the building up principle. antimony has atomic number of 51. 2.give the electron configuration of the ground state of vanadiu
We interviewed some frequent slot players and casino workers. We got these inside tips on how to increase your chances of winning. You won't want to miss thi.Información sobre el episodio 1x01 de la temporada de Boku to Misaki Sensei. Sinopsis, actores, imágenes, opiniones y críticas. ¿Que te ha parecido este capítulo?
what is the electron configuration of ag|Iba pa